Na-18 Electron Spin Resonance
Aim
To demonstrate that an unpaired electron of the organic molecule diphenyl-picrahydrazyl, or DPPH can spin up or down. Resonances are observed via induced transitions through high-frequency pumping of an external magnetic field. Resonance absorption curves can be represented with a simple dual-channel oscilloscope. This molecule is convenient in that it has one valence, unbonded electron on the second N atom. The interaction of that electron with the mean Coulomb field generated by the other electrons in the molecule ascribe an energy E0 to it. As well as having an unpaired electron, DPPH has a predominantly spin-down molecular configuration. The lifetime of the spin-up state is also short, so we’re easily able to flip the electron spin and observe the changes.
Apparatus
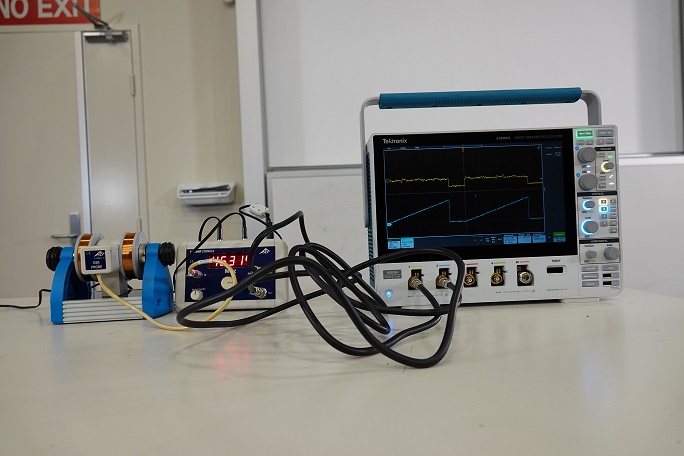
- ESR/NMR Basic Unit
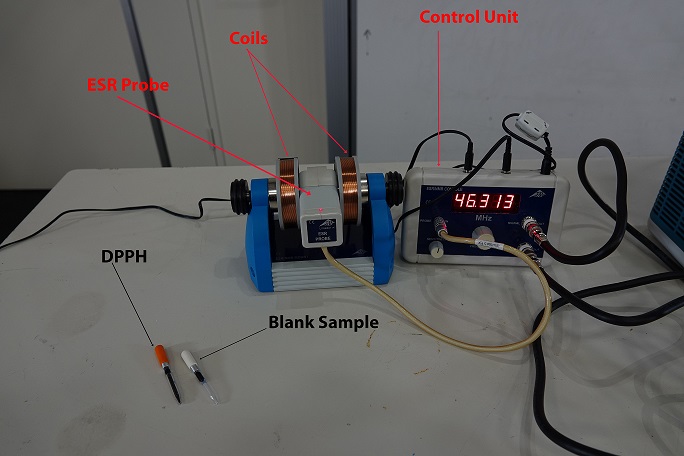
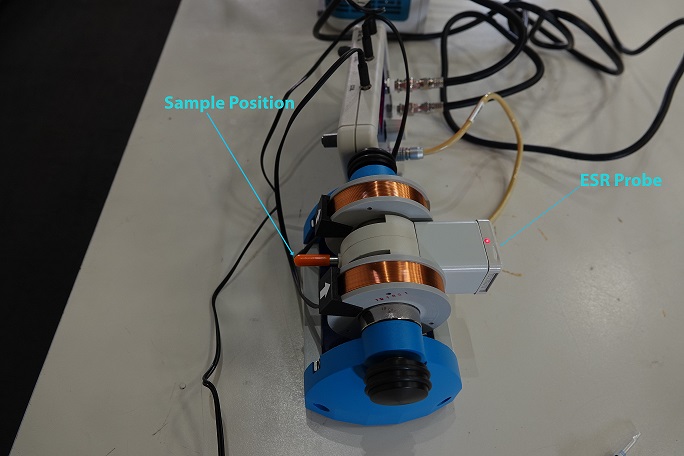
- Pair of Magnetic Coils
- Control Console
- Dual Channel Oscilloscope
- ESR Probe Unit
- Blank Sample
- DPPH Sample
Description
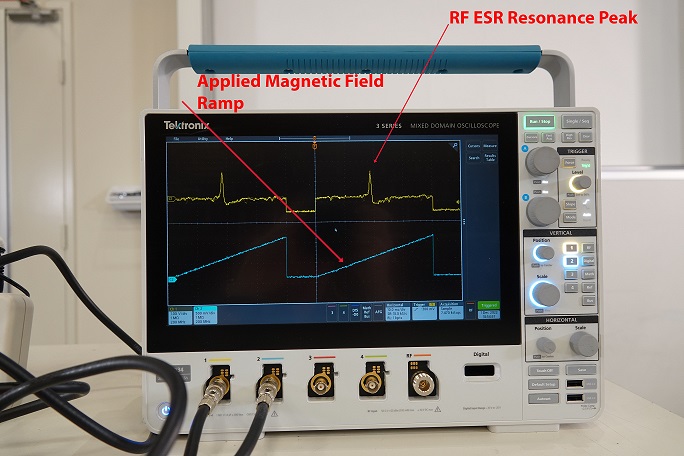
With the ESR Probe Unit connect to the Control Console, which is connected to a Dual Channel Oscilloscope. One Channel is the output of the Field of Coils, while the output is signal of the sample. When the DPPH sample is placed in the ESR Probe Unit a spike in the signal is observed when the unpaired electron is flipped when the critical magnetic field strength is reached during the sweeping of the frequency range. Which can be varied using the Control Console.When the Blank Sample is inserted in the ESR Probe Unit, a spike will not be observed in the output signal.