He-13 Gas Laws
Aim
To demonstrate Gas Law Equations using Balloons and Liquid Nitrogen.
Apparatus
- Balloons
- Cylindrical Esky
- Liquid Nitrogen
- Wooden Tongs


DESCRIPTION: The demonstration consists of placing semi inflated balloon into a cylindrical esky. The balloons are inflated so that just fit inside the cylindrical esky. Normally only 2 balloons will only fit inside the esky. By placing 1 balloon in the esky and then pouring liquid Nitrogen on the balloon, the balloon will shrink dramatically. Place another a balloon and repeat the process. It is possible to fit 10 plus balloons into the esky.
Removing the balloons from the esky, will see the balloons return to the original size prior to being cooled by liquid Nitrogen.
The Gas Laws are
Boyle’s Law
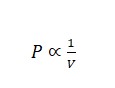
Charle’s Law
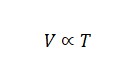
Gay-Lussac’s Law
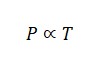
Ideal Gas Law
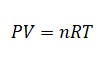
Where P is Pressure
V is Volume
T is Temperature
n is number of moles gas
R is Universal Gas Constant
By immersing the balloons in liquid Nitrogen and observing the behaviour.
The above gas laws can be demonstrated.